Design and production of Engineered Inhalable PArticles to counteract LUNG mucus barrier to drug effectiveness
Acronym: EIPA4LUNG
Duration: 24 months
Starting Date: November 2023
Funding: 225,000 €
Proposal description
Cystic fibrosis lungs are generally susceptible to colonization by Pseudomonas aeruginosa, which forms a biofilm that opposes immune system clearance and drug control. Indeed, the altered viscoelastic properties of the mucus lead to a dysfunction of the mucociliary clearance, which lowers the rate of diffusion and prevents the effective penetration of drugs, including antibiotics. An effective strategy would therefore demand for an engineered particle system capable of both counteracting the mucus barrier and fighting target bacteria. At present, there is no such product commercially available.
Combining the effect of different molecules on the mucus, we therefore propose in this project to develop, produce and characterize engineered inhalable particles able to overcome the mucous barrier and target Pseudomonas bacteria. Two strategies, with different mechanisms of action, will be pursued:
A) a chemical boost; where an agent capable of counteracting the barrier effect of the mucus envelops a core containing the antibiotic (e.g. tobramycin, levofloxacin)
B) a physical boost; where a slight effervescent particle containing the antibiotic helps the latter to reach the target bacteria.
Both types of particles will be produced using a spray drier equipped with a triple-fluid nozzle. This nozzle configuration will help to control the distribution of the sprayed components, placing the active ingredients in the envelope and/or in the core of the particle, as required by the peculiar mechanism of action.
To support this goal, ad-hoc thermodynamic and fluid-dynamic models of the process will be implemented and validated. This will help to better understand and control the effects of formulation and operating variables on the atomized particles. (It is worth noticing that the development of such models and simulations will be per-se an advancement in the knowledge of spray-drying processes.)
The produced particles will be characterized in terms of yield, drug content, morphology, density and aerodynamic properties, (after choosing a suitable device for the inhalation of powders).
Finally, to validate the proposed boost effects, both in-vitro and ex-vivo direct measurements of dissolution and permeation will be conducted. To this aim, particular emphasis will be given to elucidate the interaction between the engineered particles and the mucus, both in terms of drug mobility and enhancement of mucus microscopic dynamics.
Research units and people
Three research units are involved in the project.

Università degli Studi di Salerno (UNISA), Dipartimento di Farmacia

Consiglio Nazionale delle Ricerche (CNR), Istituto per i Polimeri, Compositi e Biomateriali

Università deli Studi di Napoli Federico II (UNINA), Dipartimento di Ingegneria Chimica, dei Materiali e della Produzione Industriale
Project organization
The project is organized in four scientific work packages.
WP1: Selection of materials and particle production (UNISA)
The development of dry powders for inhalation (DPI) requires the setting of two different elements closely interconnected: a respirable formulation (to be metered, aerosolized and deposited in high amount in the deep lung) and an opportune device for its aerosolization. The first step in the production of a powder for inhalation is the formulation of micronized particles containing the active ingredient. Given the physiological function of the respiratory apparatus, the safety of the inhaled drug has to be considered; in other words, the structural and functional integrity of the respiratory epithelium must be preserved. The first part of the project will be dedicated to a careful selection of drugs and excipients.
The particle size of the dry powder is a crucial factor in DPI formulation, with an aerodynamic diameter between 1 and 5 μm required to avoid deposition in the oropharynx and maximise deposition in the lower respiratory tract. Spray drying offers an alternative approach to the generation of dry, potentially respirable powders for systemic drug delivery. This one-step process can be used to produce dry powders from solutions, suspensions or emulsions, with greater control over particle size, morphology and powder density than destructive methods of powder production, such as micronisation.
The need to produce precisely engineered particles prompted us to select the triple-fluid pneumatic nozzle to obtain the micronized droplets in the drying chamber of the spray. Two strategies will be proposed: (i) using an agent capable of modifying the mucus barrier effect (defined as a chemical approach), (ii) using an ingredient capable of producing a local effervescence (defined as a physical approach). In the following are reported some details of both approaches.
For both strategies, engineered particles will be prepared using a mini spray drier equipped with a triple-fluid nozzle, pumping aqueous or hydroalcoholic solutions (water/ethanol or water/isopropyl alcohol) selected according to drugs and excipient solubilities. The addiction of dispersibility agent, able to improve powder aerodynamic properties will be also investigated. To optimize the system in terms of composition and dimensions of the micro-powders, it is necessary to identify the operating conditions of the process: flow rates, concentrations, temperatures, etc. In this regard, thermodynamic and fluid dynamic analysis of the triple-nozzle will be carried out in WP2 and WP3.
WP2: Equation of State implementation – Phase diagrams (CNR)
During drying the mixture passes through a series of concentration states and separate phases, which ultimately determine the characteristics of the obtained powder. Knowledge of drying curves, i.e., the curves describing the evolving compositions of the liquid phase, allows to identify the homogeneous liquid regions, the miscibility gaps and the glass transitions encountered by the system during the process. To design a robust formulation process, the knowledge of the phase diagram and concentration change of the mixture during drying is therefore an indispensable prerequisite. Such information allows optimizing the choice of solvent and the process conditions to obtain the specifics required. Predictions of the properties of mixtures under certain operating conditions are obtained through Equation of State (EoS) models.
We implement a thermodynamic approach that describes the phase changes during drying. This approach includes the generation of phase diagrams and the calculation of drying curves. Moreover, the influence of factors such as the initial concentration of the feed solution, liquid-liquid phase separation, crystallization and drying temperature, will be also considered. Raman spectroscopy measurements will be performed to continuously monitor the concentrations of active ingredient, polymer, excipients and solvent in the liquid phase. The mixtures will be prepared in different ratios and different solvents.
Knowledge of phase diagrams constitutes only a pre-requisite to correctly modeling the particle formation during a drying process. The drying curves are strictly tangled to the specific drying technique. In the case of spray drying, these curves are the result of several rather complex elementary processes, such as: the atomization of the droplets, the transport of the particles, the evaporation of the droplets and the interaction between particles and / or droplets and / or walls of the dryer. In this scenario, computational fluid dynamics (CFD) (carried out in WP3) turns to be a useful tool capable of predicting, or at least restricting, the range of parameters for obtaining the required specifications.
WP3: Fluid-dynamic modelling and simulation (UNINA)
The spray-drying process involves two immiscible phases, one liquid and one gas, where the liquid is pumped at high pressure through a nozzle and is atomized in small droplets through the effect of the air within a short distance from the nozzle exit. The formed droplets may further break-up, coalesce and evaporate before reaching the outlet. For the triple-nozzle investigated in this project, two liquid phases are considered along with the gas so that a multiphase system made of three fluids need to be modelled. One liquid contains an active principle whereas a chemical boost molecule (A) or physical boost agents (B) are dissolved in the second liquid. The modeling and simulation of the spray-drying process include multiple sequential stages: i) the investigation of the liquid–gas interaction inside and at the nozzle exit, ii) the break-up of the liquid and the formation of droplets, iii) the dynamics of the droplets in the open environment, iv) the transport of the species (active principle, excipient/polymer) inside the droplet, v) the evaporation of the droplets and the precipitation of the dissolved species to form particles.
The dynamics of the fluids (liquids + air) inside the nozzle, their hydrodynamics interaction at the nozzle exit and the atomization stage will be simulated by combining the Volume-Of-Fluid (VOF) and the Discrete Phase Model (DPM) methods. The evaporation of the formed droplets and the consequently precipitation step will be treated by modelling the dynamics of a single liquid droplet moving in an ambient air under conditions given by the VOF-to-DPM results. The single droplet model will be applied to a population of generated droplet from atomization simulations, characterized by different size and species concentration. The outcomes of the simulations will be used to validate the numerical tool by comparing the simulation data with the experimental ones obtained by UNISA such as the droplet/particle size distribution and the precipitation rate.
WP4: Characterizations (UNISA and CNR)
In order to follow the progress of the process and at the same time compare it with the fluid dynamics simulations, some process observables will be acquired. In addition to the characterization of the powders produced needed for the model validation (WP3), the process temperature and the pressure inside the drying chamber will also be measured. The pressure inside the precipitation chamber will be acquired through two transducers positioned near the nozzle and at the end of the chamber, before entering the cyclone.
Based on the expertise on the formulation of powders for inhalation of UNISA group, engineered particles will be tested in terms of yield of the process, morphology (Scanning Electron Microscopy), size distribution (Laser Scattering), density (helium pycnometer, or tapped density test), drug content (UV or HPLC) and stability. For powders promoting a physical boost to the penetration of the active ingredient into the mucus, the released effervescence when in contact with fluids will be evaluated. Moreover, during the pharmaceutical development, the in vitro respirability of the produced powders will be initially assessed by means of the Single Stage Glass Impinger, which allows a first and rapid screening of powders aerodynamic behaviour.
The efficacy of the drug is strictly connected to the overcoming of the mucosal barrier. In this regard, in-vitro and ex-vivo direct measurements on the permeation of the active ingredient will be conducted.
A relatively new method of dynamic light scattering, named photon correlation imaging PCI, was recently introduced to solve the dynamics of soft matter in space and time. This technique allows, in fact, to investigate the slow dynamics of different soft materials, ranging from deformable bubbles to the gel network of colloidal particles. Recently the technique has also been applied to resolve the spatio-temporal correlation dynamics within a porcine gastric mucus. We propose, for the first time to the best of our knowledge, to investigate the effect of the excipient on mucus by PCI. To do this, a layer of mucus (sputum or porcine-gastric) will be covered by the developed particles and its microscopic dynamics will be monitored, in back scattering configuration both, spatially and temporally. The collected dynamics will give insights on the effect of powder on the mucus local mobility.
Results
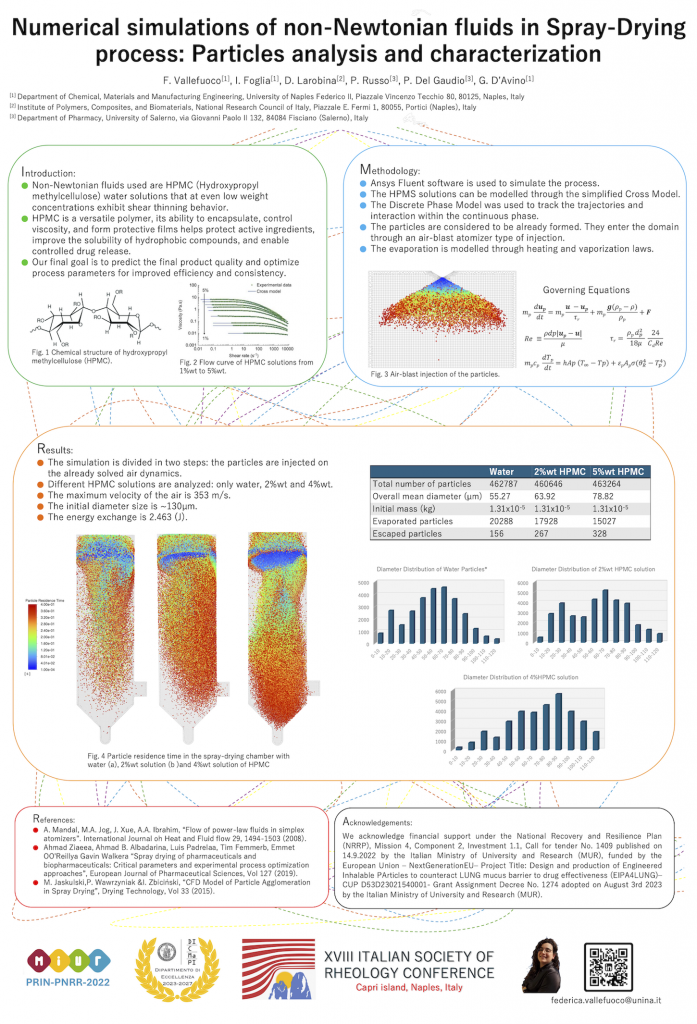
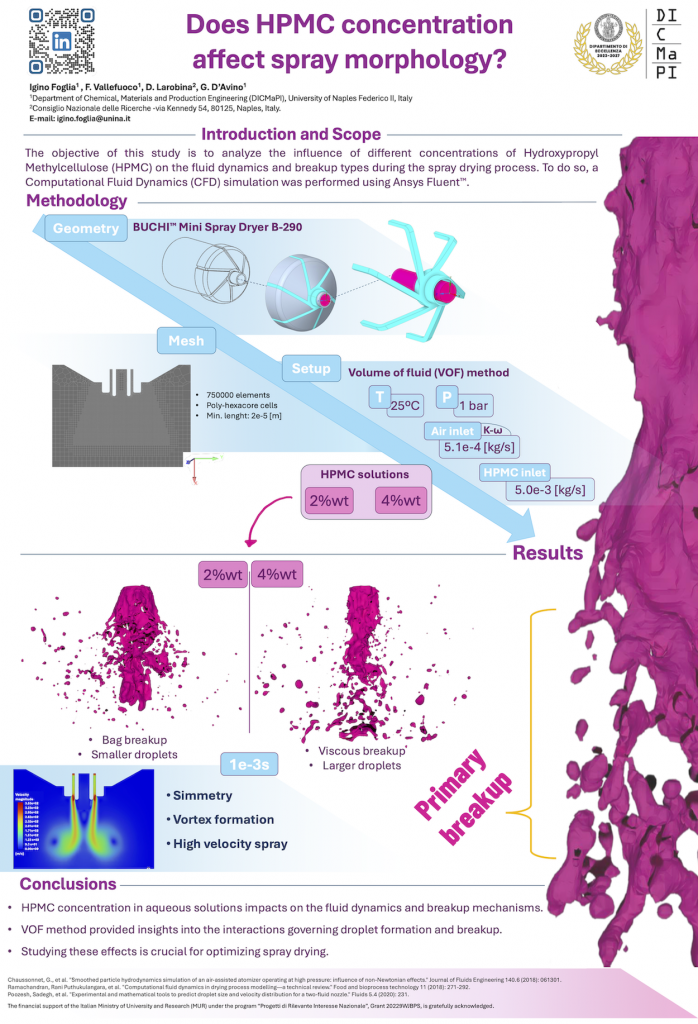
Spray drying modeling and simulation
Computational fluid dynamics simulations have been carried out to model the spray drying process with an innovative triple-fluid nozzle. The velocity and pressure fields are accurately evaluated in the nozzle and in the drying chamber. Two different approaches to model and simulate the spray-drying phenomenon in the chamber have been employed. In the first approach denoted as “CFD-DPM”, the fluid dynamics of the air in the chamber are considered without accounting for the injection of the liquid. In a second step, the already formed droplets are injected close to the atomization nozzle and their trajectories are tracked inside the chamber, including the evaporation process. Such a technique allows to predict the evaporation phenomenon in the spray-dryer along with the temperature profiles and droplet size distribution in a reasonable computational time. On the other hand, since atomization is not modelled, the initial size distribution of the droplets must be provided. To this aim, a size distribution based on the measurements of the produced powder is considered.
In the second approach denoted as “VOF-DPM”, the atomization process is simulated by tracing the evolution of the liquid interface. This technique requires much more computational effort but can provide the droplet size distribution of the atomized droplets. Such a method is also applied to non-Newtonian fluids that are typically used in spray-drying processes.
For more information click on the posters below
Powder production and characterization
Through the optimization of spray dryer parameters and feed solution compositions, effervescent micronized powders were successfully obtained using an innovative triple-fluid nozzle. The addition of leucine further enhanced the powder characteristics. The promising results suggest the potential use of this powder for lung drug administration, offering a new perspective in treating conditions where mucus viscosity and elasticity often compromise the effectiveness of inhaled medications.
Furthermore, the produced engineered powders are proven to be beneficial in treating conditions such as cystic fibrosis by addressing excessive mucus viscosity in the respiratory pathways. These results should be confirmed and supported by drug permeation studies through simulated mucus, to verify the in vitro effectiveness of the proposed particles.
For more information click on the posters below
Dissemination activities
A workshop to disseminate the project results was organized at the University of Salerno on December 15, 2025, in collaboration with two additional PRIN-PNRR projects. Three presentations were delivered for the EIPA4LUNG project: one by UNINA-CNR on modeling and simulation activities, one by UNISA on the experimental work, and one by the invited external company INDENA, which addressed scale-up challenges in industrial pharmaceutical applications.
For more information click on the flyer below